9+ Does Japan Require Iec-60601 4Th Edition
Web Manufacturers must plan for the worst and hope for the best. Web The risk management process now part of the IEC 60601-1-2 4th edition requires assessment of risk resulting from reasonably foreseeable electromagnetic disturbances.
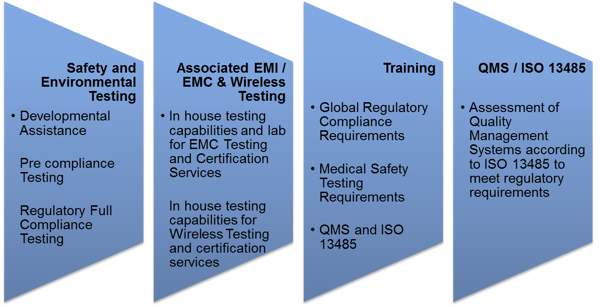
Ensuring Medical Device Effectiveness And Safety
General requirements for basic safety and essential.

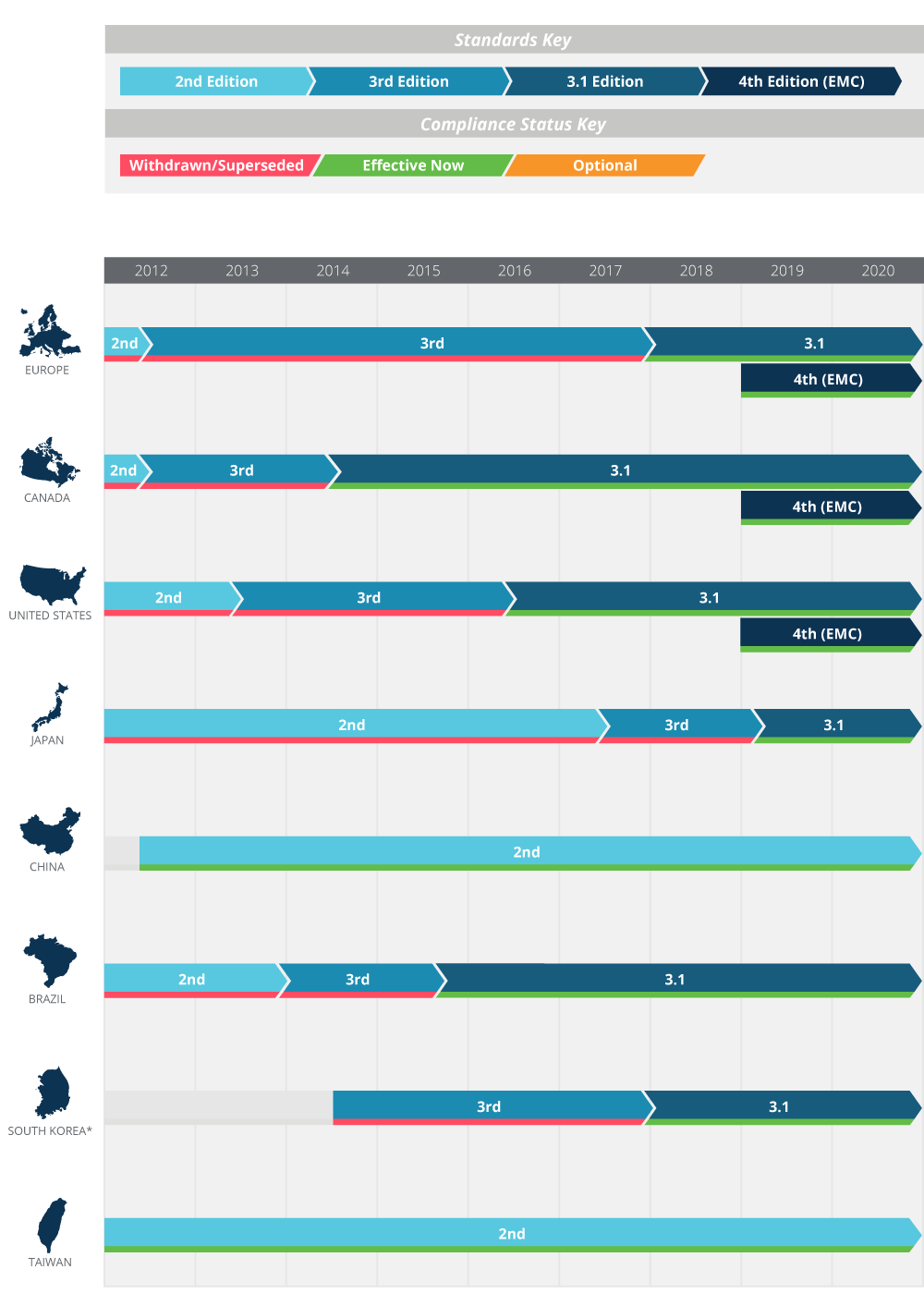
. Web Emergency Medical Services are automatically classified as the Home Healthcare Environment per clause 81 of IEC 60601-1-2 4th edition. Web The USA Canada Japan Australia and New Zealand have not yet set transition dates for their national versions of this latest edition 60601-1 but the national versions published. 41 の概要 2 適用範囲 IEC 60601-1-2 は医用電気機器ME機器や医用 電気システムMEシステムに適用され医用電気 機器はIEC 60601.
Web The most recent addition of IEC 60601-1-2 has been released. Web There is much debate surrounding the answer to this question with many experts feeling that Japan does indeed require the IEC-60601 4th edition in order to ensure safety and compliance with international standards. Web Manufacturers must submit risk analysis documentation for both normal and abnormal use of their equipment and systems.
Web The 4th edition requires the basic safety and essential performance of the medical device by maintained in the presence of the various electromagnetic. Web The fourth edition IECEN 60601-1-2 4th Edition will become a mandatory standard covering safety for medical devices on December 31 201812 As with any new. IEC 60601-1 edition 32 will cause manufacturers to abandon selling medical devices to countries.
Web An expert discusses what medical device manufacturers need to keep in mind as the compliance date for the fourth edition of the IEC 60601-1-2 standard approaches. Web IEC 60601-1-22014A12020 ed. The IEC 60601-1-22020 ed41 features some new tests as well as some modifications to some existing.
Web The standard governing electromagnetic compatibility EMC in medical devices IEC 60601-1-2 4th edition has been in effect for several years. The standard also indicates. Date of Withdrawal of EN 60601-1-22007 3rd.
This is clear even from a quick look at the table of contents. The presentation will address the following topics. Web IEC60601 41版とは2020年の年末ごろに発表となった新しいバージョンのEMC規格です 4版の修正条項第1条によって修正され41版と呼ばれています 代表的な変更点は.
This standard is often referred to as the. Web the fourth edition replaces the life support and non-life support classifications used in the third edition with three intended use environments. Web The 4 th edition of IEC 60601-1-2 differs significantly from the previous version.
Web IEC 60601-1-2 Edition 4 will soon be mandatory and this means dramatic changes for the medical electronics industry. Web The original premise of the standard is mirrored by its full title IEC 60601-1 - Medical electrical equipment - Part 1. 3 1685 Rating Highest rating.
This post was originally published October 31 2017 and updated on February 12 2019 to reflect the latest timeline for adoption of IEC 60601-1 worldwide. Web IEC 60601 is a widely accepted series of international standards for the Basic Safety and Essential Performance of Medical Electrical Equipment. Web The main IEC 60601-1 standard referred to in Europe as EN 60601-1 and in Canada as CSA 60601-1 is an umbrella for numerous subsidiary standards variously.
Web Since March 1 st 2014 Japans MHLW and PMDA recognizes the national equivalent of IEC 60601-1 2 nd edition IEC 60601-12005 3 rd edition and 60601.
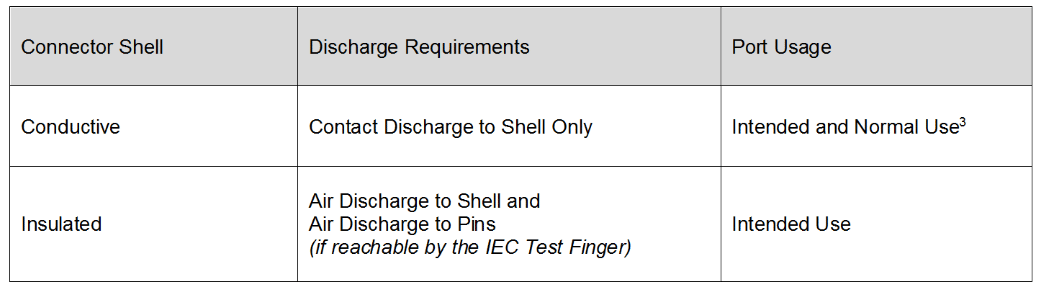
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Be Prepared For The 4th Edition Of The Iec 60601 1 Medical Standard

Electrical Safety Testing For Medical Devices And Ivds Tuv Sud
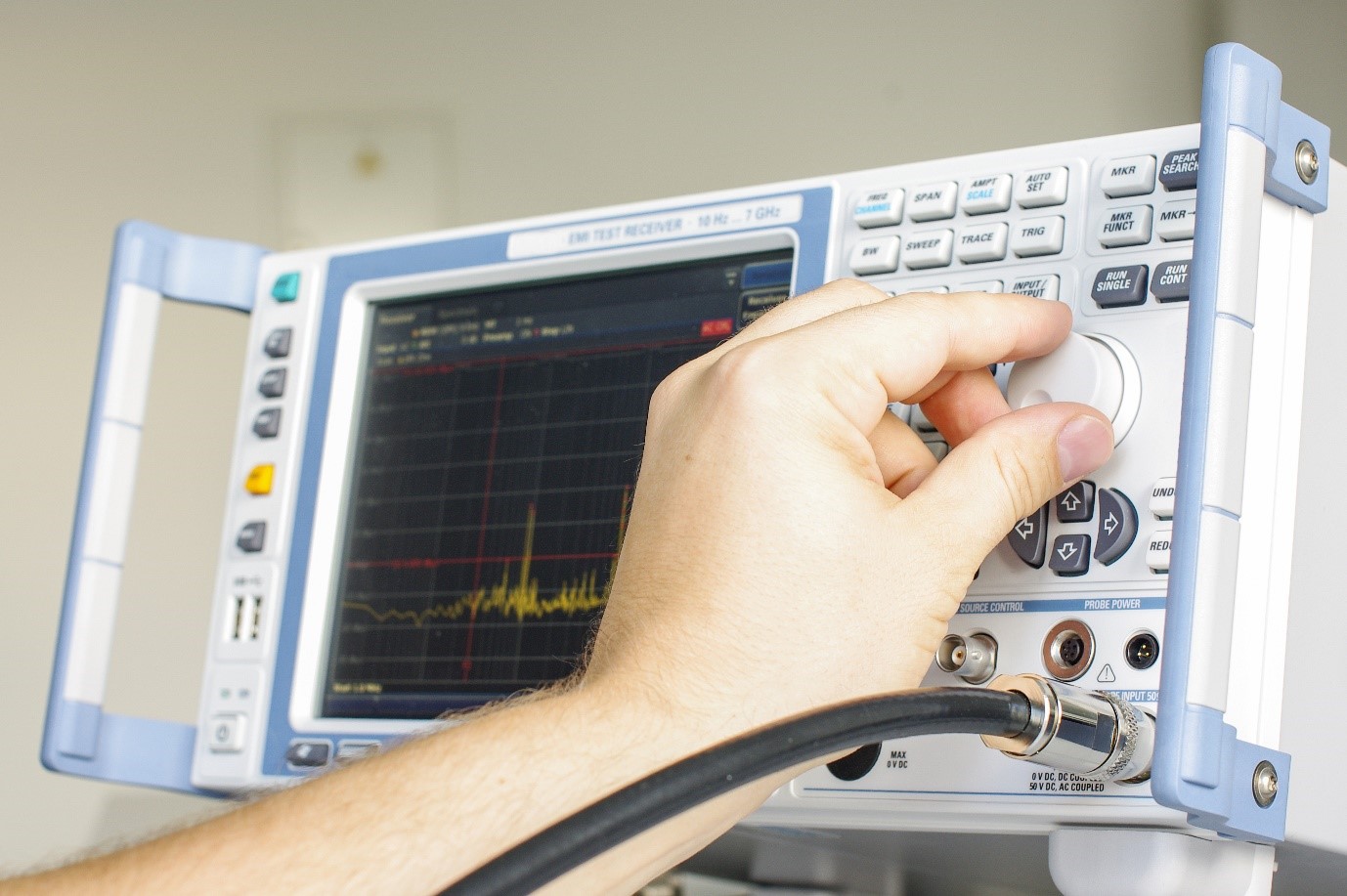
Ensuring Medical Device Effectiveness And Safety

Medizinische Iec 60601 1 Designstandards Fur Netzteile Cui Inc

Emc Requirements For Medical Power Supplies Medical Design And Outsourcing

Iec 60601 1 Medical Design Standards For Power Supplies Cui Inc

Pdf Medical Electrical Equipment Part 1 General Requirements For Basic Safety And Essential Performance Richard Um Academia Edu

Iec 60601 3rd Edition Adopted In China Sesec Iv Archive 2016 2020
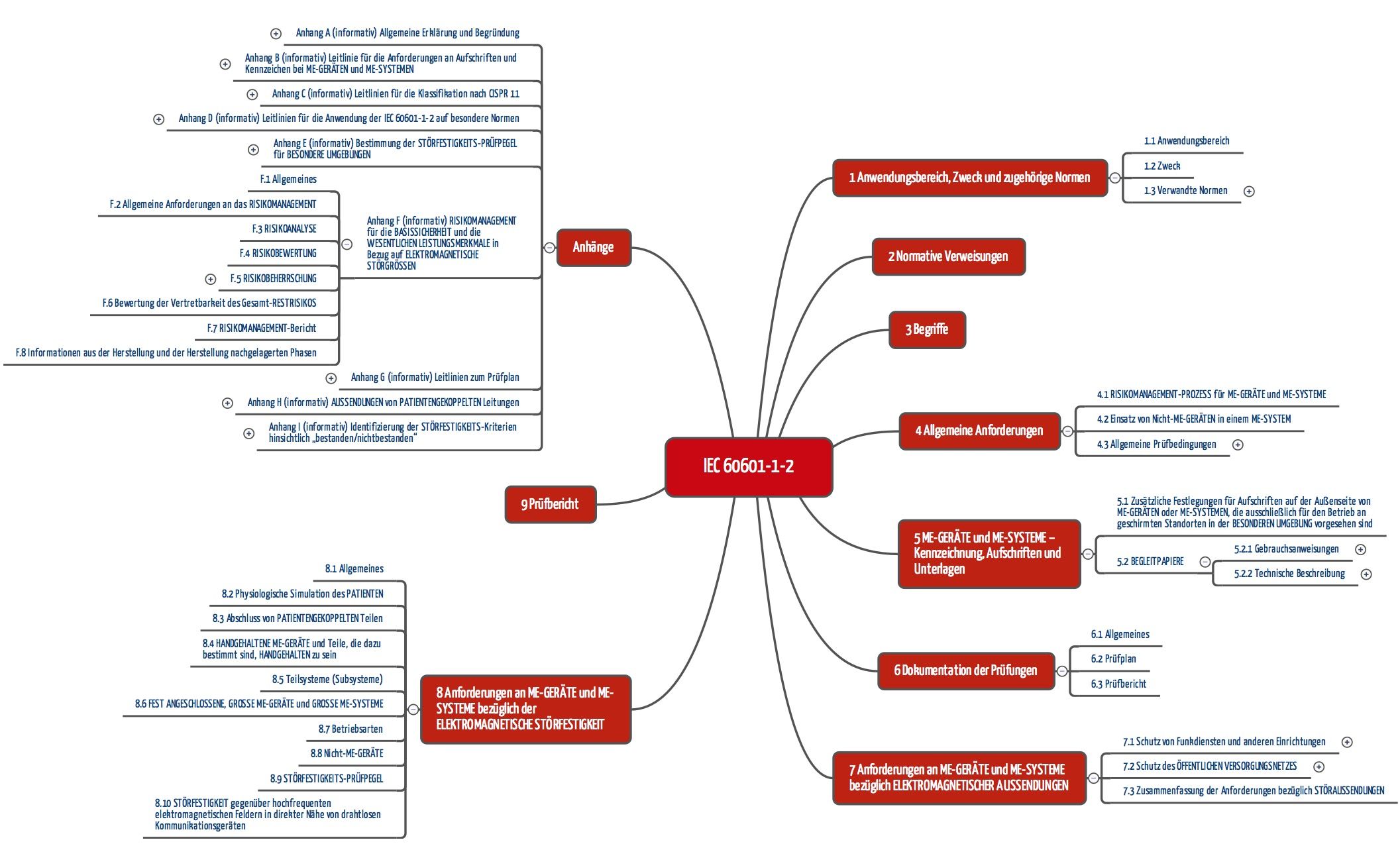
Iec 60601 1 2 Edition 4 Elektromagnetische Vertraglichkeit 2016

Iec 60601 1 2005 End Of Transition Periods Of The Amendment 1 2012 Tuv Sud

Elexes Blog Iec 60601 1 Evolution Of Electrical Safety
Iec 60601 1 Medical Design Standards For Power Supplies Cui Inc

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn

Iec 60601 Standards Series Electrical Medical Devices Manufacturers

Iec 60601 1 2 2014 Amd1 2020 Amendment 1 Medical Electrical Equipment Part 1 2 General
Iec 60601 1 Medical Design Standards For Power Supplies Cui Inc